EMPOWERING COLLABORATIVE AND
DIVERSE NEUROSCIENCE RESEARCH,
EDUCATION AND OUTREACH AT UCLA.
Joint Seminars In Neuroscience
UP NEXT:
May 14, 2024
“From Moments to Engrams: Artificially Manipulating Memories in Healthy and Pathological States”
Department of Psychological and Brain Sciences, Boston University
Faculty Hosts: Michael Wells
In-Person Event
12:00 PM
NRB 132 Auditorium
For more information, please contact the BRI Administrative Office at BRI@mednet.ucla.edu.
Upcoming Events/Seminars
- 9:00am
Neurology Grand Rounds – David Brody, M.D., Ph.D.
- 12:00pm
CTSI Distinguished Speaker Seminar – Joseph Takahashi, Ph.D.
- 12:00pm
Broad Stem Cell Research Center (BSCRC) Research Seminar – KristenBrennand, Ph.D.
- 12:00pm
Integrative Center for Neurobehavioral Genetics (ICNG) Seminar Series – Andy Dahl, Ph.D.
- 5:00pm
Friends of the Semel Institute Showcase – Project UnLonely
- 9:30am
Integrative Center for Learning and Memory (ICLM) Journal Club – Tanya Gupta, Ph.D.
- 12:00pm
Joint Seminars in Neuroscience (JSN) Series – Anna V. Molofsky, M.D., Ph.D.
- 8:00am
Ecological Medicine & Psychedelic Studies Initiative (PSI) Symposium – “Cultivating Connections: Ecological Medicine Meets Psychedelic Therapies”
- 9:30am
Integrative Center for Learning and Memory (ICLM) Journal Club – Deepak Singla
- 12:00pm
Integrative Center for Neural Repair (ICNR) Seminar Series – FannyEtienne, Ph.D.
- 3:30pm
Synapse to Circuit (S2C) Seminar Series – Andre Sousa, Ph.D.
- All Day
Pint of Science 2024
- 12:00pm
Intellectual and Developmental Disabilities Research Center (IDDRC) Seminar Series – Rujuta B. Wilson, M.D., M.S.
- All Day
Pint of Science 2024
- 12:00pm
Joint Seminars in Neuroscience (JSN) Series – Steve Ramirez, Ph.D.
- 3:00pm
Integrative Center on Addictions (ICA) Seminar Series – Golnaz Tabibnia, Ph.D.
- 5:00pm
Integrative Center for Neurobehavioral Genetics (ICNG) Journal Club – Greta Gerdes
- All Day
Pint of Science 2024
- 9:00am
Neurology Grand Rounds – Charlotte Sumner, M.D.
- 12:00pm
Stein Eye Institute (SEI) Research Seminar Series – Joel Zylberberg, Ph.D.
- 12:30pm
Immunology, Inflammation, Infection and Transplantation (I3T) Seminar Series – David M. Ashley, M.B.B.S. (Hon.), F.R.A.C.P., Ph.D.
- 3:30pm
20th Jeffrey Lecture on Cognitive Neuroscience – Doris Tsao, Ph.D.
- 10:00am
Jeffrey Lecture Specialized Talk – Doris Tsao, Ph.D.
- 3:30pm
Neuroimaging Affinity Group Colloquium – Stephanie Noble, Ph.D.
- 9:00am
2024 Neurobehavioral Genetics Computational Workshop
- 10:00am
STAND ALACRITY Center Seminar Series – Lauren Brookman-Frazee, Ph.D.
- 12:00pm
Joint Seminars in Neuroscience (JSN) Series – 35th Annual H.W. Magoun Distinguished Lecture – TBD
- 12:00pm
CTSI Distinguished Speaker Seminar – Aaron Gitler, Ph.D.
- 3:00pm
25th Annual Neuroscience Poster Day
- 12:00pm
Integrative Center for Neurobehavioral Genetics (ICNG) Seminar Series – Sarah Kocher, Ph.D.
- 9:30am
Integrative Center for Learning and Memory (ICLM) Journal Club – Peyman Golshani, M.D., Ph.D.
- 12:00pm
Laboratory of Neuroendocrinology (LNE) Seminar Series – Naoki Yamanaka, Ph.D.
- 12:00pm
Integrative Center for Neural Repair (ICNR) Seminar Series – William Lowry, Ph.D.
- 3:30pm
Synapse to Circuit (S2C) Seminar Series – Ricardo Frausto
- 12:00pm
Joint Seminars in Neuroscience (JSN) Series – Roy Sillitoe, Ph.D.
The UCLA Brain Research Institute (BRI) is a catalyst for education, outreach, and research collaborations among current and future scientists, engineers and clinicians who seek to understand the healthy and diseased brain.
UCLA is rated the #1 Public University in the country.
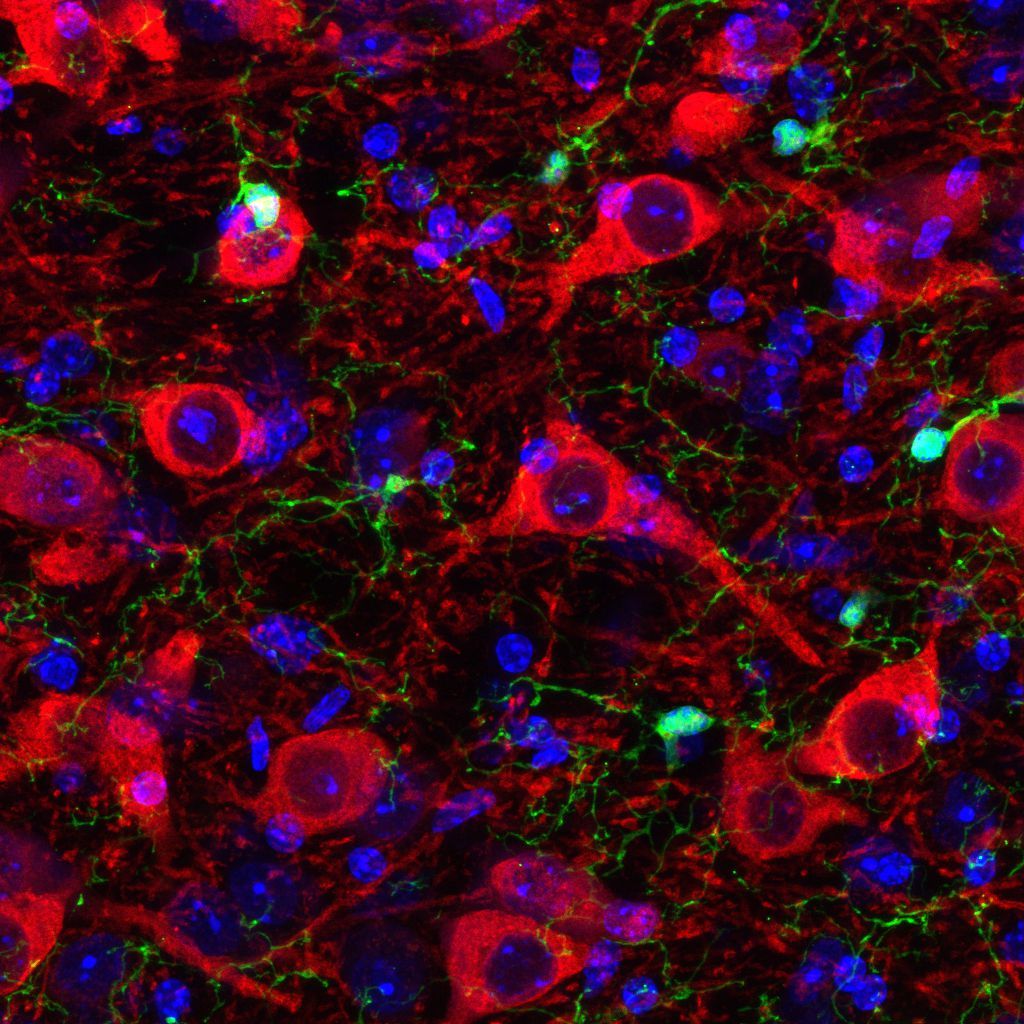
Image of the Month
May 2024 – Immunohistochemical analysis of the mouse ventral tegmental area. Green is microglia which express EGFP under the endogenous cx3cr1 locus and is amplified by GFP antibody. Red is dopamine neurons labeled with TH, and blue is nuclei labeled with DAPI.
Image by Keionna Newton from the lab of Dr. Lindsay De Biase.
#1 Educational Program
UCLA is ranked the #1 public education program in the country by US News. Programs are diverse, affordable, and mission driven.
Life Changing Research
The BRI provides multiple mechanisms to invigorate research programs and support collaborations bringing together investigators from complementary fields. These mechanisms include
Outreach That Impacts Our Communities
The BRI aims to extend scientific knowledge into the community and inspire elementary and secondary school students to explore a career in neuroscience